Suprasorb® P + PHMB
Antimicrobial PU Foam Dressing
Suprasorb P + PHMB with perforated wound contact layer impresses with its reliable absorption of exudate. The PHMB incorporated in the foam is effective both in the dressing and in the wound. This is why Suprasorb P + PHMB is classified as a Class III medical device.
The material is soft and conformable. The special surface structure of the Suprasorb P + PHMB wound contact layer is gentle on easily injured surfaces like healing wounds. The semipermeable PU carrier layer maintains a moist wound healing environment. Impermeable to bacteria and water.
Suprasorb P + PHMB is suitable for use under compression bandages.
Fields of application
- moderately to heavily exuding wounds
- for wounds that are infected and at risk of infection
- for superficial wounds
Wound healing stages:
- Exudation phase
- Granulation phase
- Epithelialisation phase
Indications
- leg and foot ulcers
- decubital ulcers
- diabetic ulcers
- postoperative surgical wounds
Properties
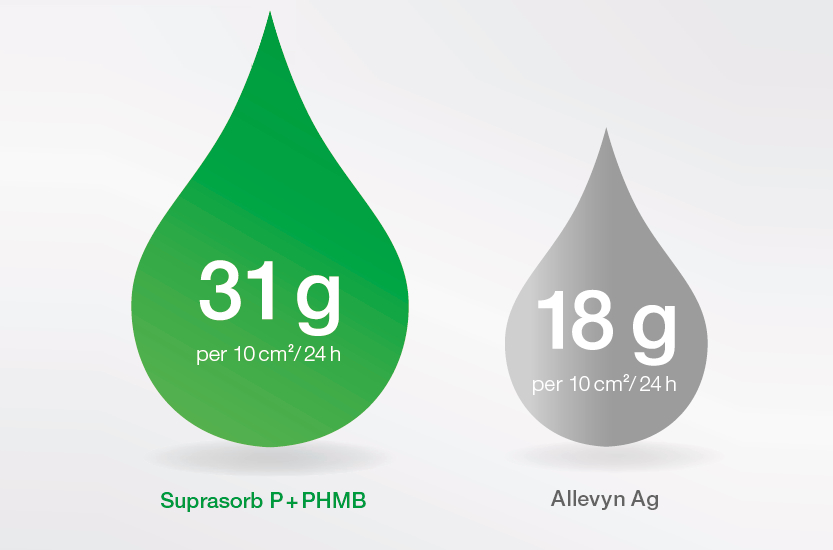
- reliable exudate management
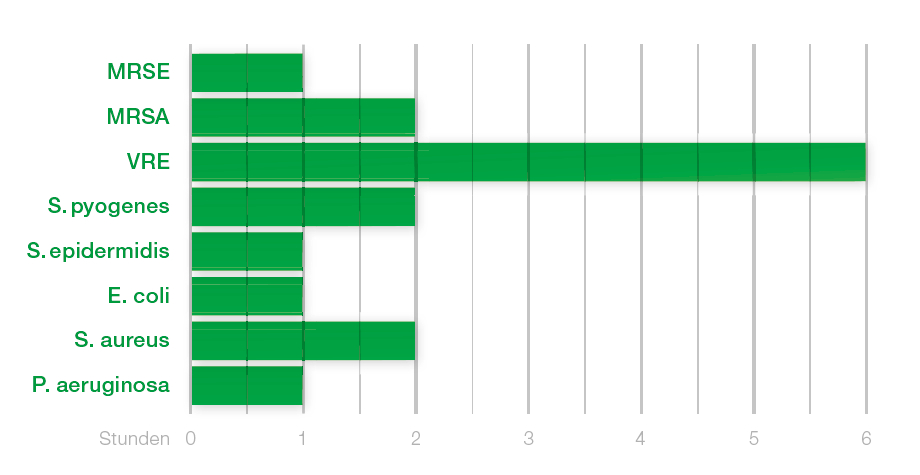
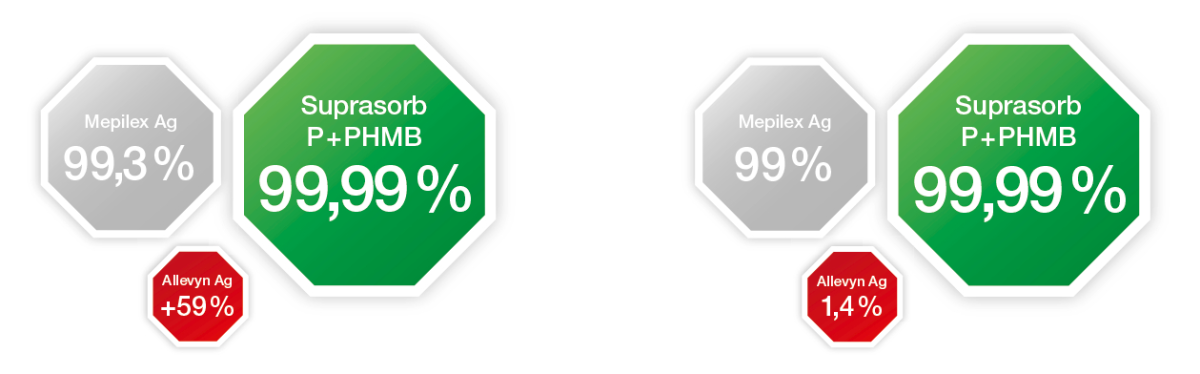
- broad antimicrobial action
- rapid and prolonged effect
- with a perforated wound contact layer
- water- and bacteria-proof
To note
- Suprasorb P + PHMB can be used as a secondary dressing with wounds at risk of infection and infected wounds within the scope of a regular antimicrobial therapy regimen.
- When dry, Suprasorb P + PHMB can easily be cut to the size of the individual wound.
Report AMS P2337R, data on file
Report AMS OT50 rev.5, data on file
Report AMS P2999R, data on file, relates to Ps. aeruginosa and Staph. aureus, effective for 7 days
Report AMS LD017, P2412, data on file