Suprasorb® P sensitive
Silicone Foam Dressing
Suprasorb P sensitive is a silicone foam dressing especially for sensitive skin61) . Its multilayer construction provides dynamic exudate management for an optimal moist wound environment62) . The silicone wound contact layer minimises the risk of adhesion to the wound and pain for the patient during dressing changes63) . The outer protective film is waterproof and repels bacteria, while also being permeable to water vapour.
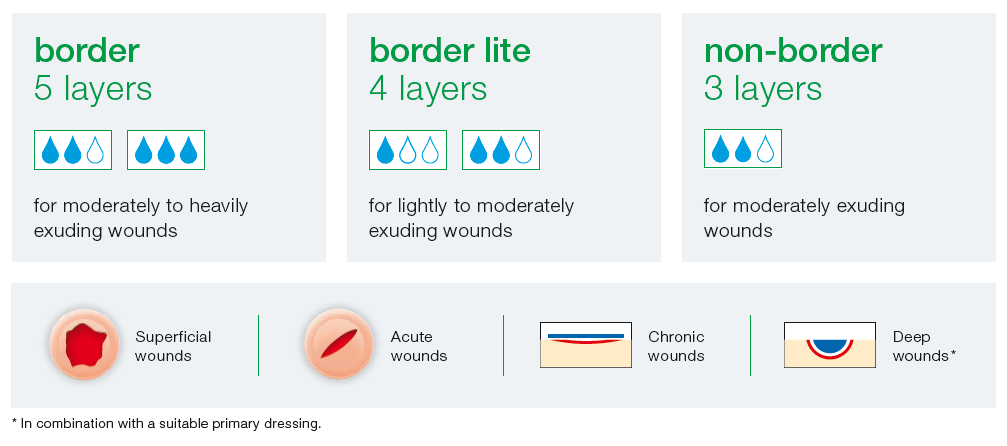
Suprasorb P sensitive is available in three versions depending on the degree of exudate: “border”, “border lite” and “non-border”
Versions
heel (with super-absorbent core)
sacrum (with super-absorbent core)
multisite border (with super-absorbent core)
multisite border lite (without super-absorbent core)
tracheo (without super-absorbent core)
Ordering information
Suprasorb P sensitive
| Dimensions (cm) | Wound pad (cm) | REF | SC/TC |
|---|---|---|---|
| border (with super-absorbent core) | |||
| 8.5 x 7.5 | 5 x 4.5 | 139068 | 10/200 |
| 10 x 10 | 6.2 x 6.2 | 139061 | 10/200 |
| 12.5 x 12.5 | 8.5 x 8.5 | 168062 | 10/200 |
| 15 x 15 | 10.5 x 10.5 | 168069 | 10/150 |
| 30 x 10 | 30 x 10 | 146240 | 5/200 |
| 20 x 20 | 15 x 15 | 139070 | 10/100 |
| border lite (without super-absorbent core) | |||
| 5 x 5 | 2.5 x 2.5 | 139080 | 10/240 |
| 10 x 5 | 6 x 2.5 | 139081 | 10/200 |
| 8.5 x 7.5 | 5 x 4.5 | 139086 | 10/200 |
| 10 x 10 | 6.2 x 6.2 | 139083 | 10/200 |
| 20 x 10 | 15 x 5 | 139088 | 10/150 |
| non-border (without super-absorbent core) | |||
| 5 x 5 | 139350 | 10/240 | |
| 7.5 x 7.5 | 139455 | 10/150 | |
| 10 x 10 | 139351 | 10/100 | |
| 20 x 10 | 139352 | 10/150 | |
| 15 x 15 | 139353 | 10/100 | |
| 20 x 15 | 139456 | 10/100 | |
| 20 x 20 | 139354 | 10/100 | |
| sacrum (with super-absorbent core) | |||
| 17.5 x 17 | 12.5 x 12 | 139355 | 10/100 |
| 23 x 23 | 17 x 16.5 | 139556 | 10/100 |
| heel (with super-absorbent core) | |||
| 25 x 23.5 | 19 x 17.5 | 139356 | 10/100 |
| multisite border (with super-absorbent core) | |||
| 15 x 12 | 10 x 7 | 168357 | 10/100 |
| 7.5 x 9.5 | 3.5 x 5.5 | 168075 | 10/300 |
| 16 x 20 | 11.5 x 15.5 | 168076 | 10/200 |
| multisite border lite (without super-absorbent core) | |||
| 7.5 x 9.5 | 3.5 x 5.5 | 168077 | 10/300 |
| tracheo non-border (without super-absorbent core) | |||
| 10 x 9 | 9 x 10 | 146244 | 10/100 |
Fields of application
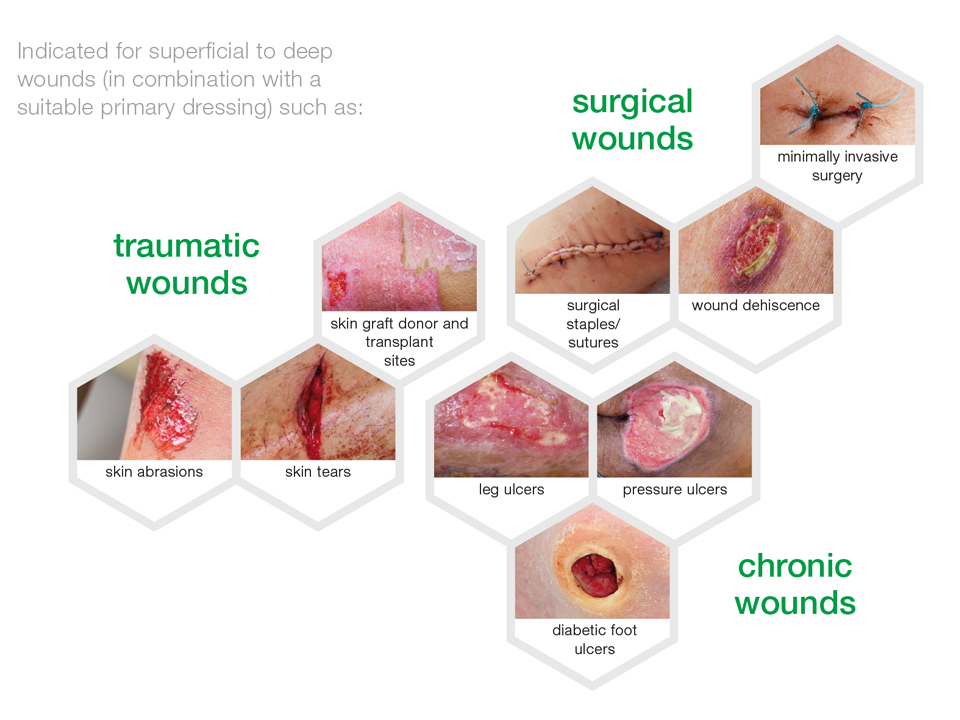
- For treating the following wounds:
- acute and chronic
- moderately to heavily exuding
- superficial or deep (where necessary, for deep wounds apply a packing rope as primary dressing)
- Indications:
- pressure ulcers
- leg and foot ulcers
- traumatic wounds
- surgical wounds*)
- skin tears
- upon medical advice, can be used as a secondary dressing for infected wounds and wounds at risk of infection
- Suprasorb P sensitive tracheo non-border can be used for covering skin areas around tracheal cannulae and other drains.
*Suprasorb P sensitive border and Suprasorb P sensitive border lite
- Wound healing stages:
- exudation phase, granulation phase and epithelialisation phase
Properties
- Silicone wound contact layer is suitable for fragile skin.
- Minimises pain to the patient and trauma to wound and the surrounding skin when the dressing is removed or repositioned.
- Efficient exudate management reduces the risk of maceration and maintains moist environment.
- Shower-proof.
- Easy application with 2 or 3 liners, which allows to not touch the adhesive with gloves.
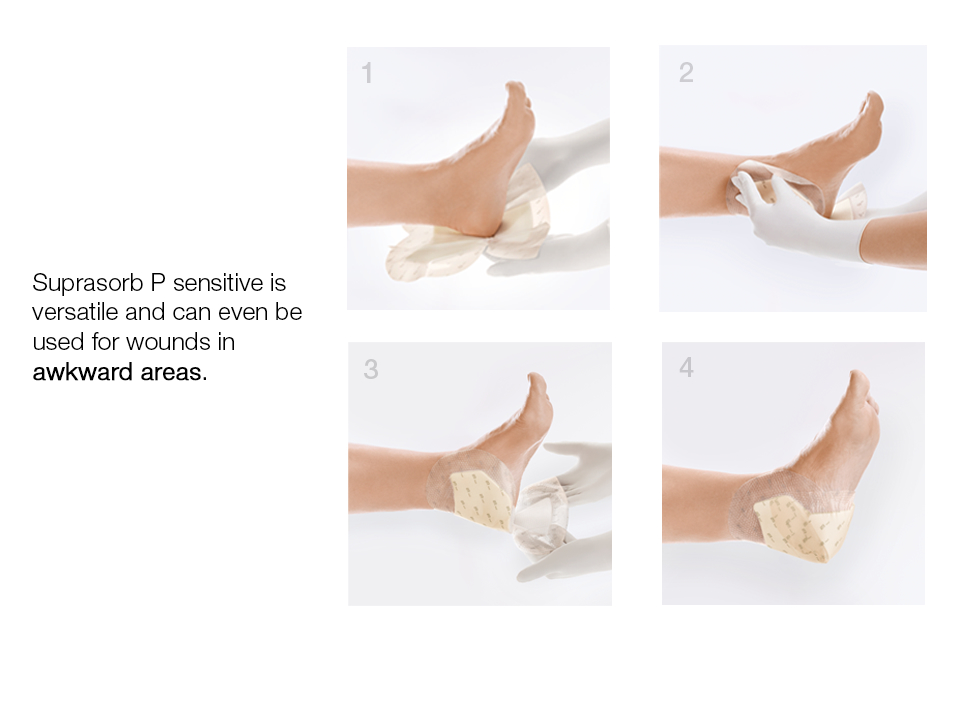
- Great conformability in awkward areas.
- Gentle on the skin.
- Can be used as part of a prophylactic therapy to prevent pressure ulcers.**)
- Saves time thanks to longer dressing change intervals.
**Suprasorb P sensitive border
Pukki, T., Tikkanen, M., & Halonen, S. (2010). Assessing Mepilex Border in post-operative wound care. Wounds UK,6(1), 30-40.
Barrows, C. (2009). Enhancing Patient Outcomes-Reducing the Bottom Line:
The Use of Antimicrobial Soft Silicone Foam Dressing in Home Health. Home Healthcare Now, 27(5), 279-284.
Davies, P. (2008). Evidence review: the clinical benefits of Safetac technology in wound care. Journal of wound care.