Suprasorb® Liquacel*
Hydroactive Fibre Dressing
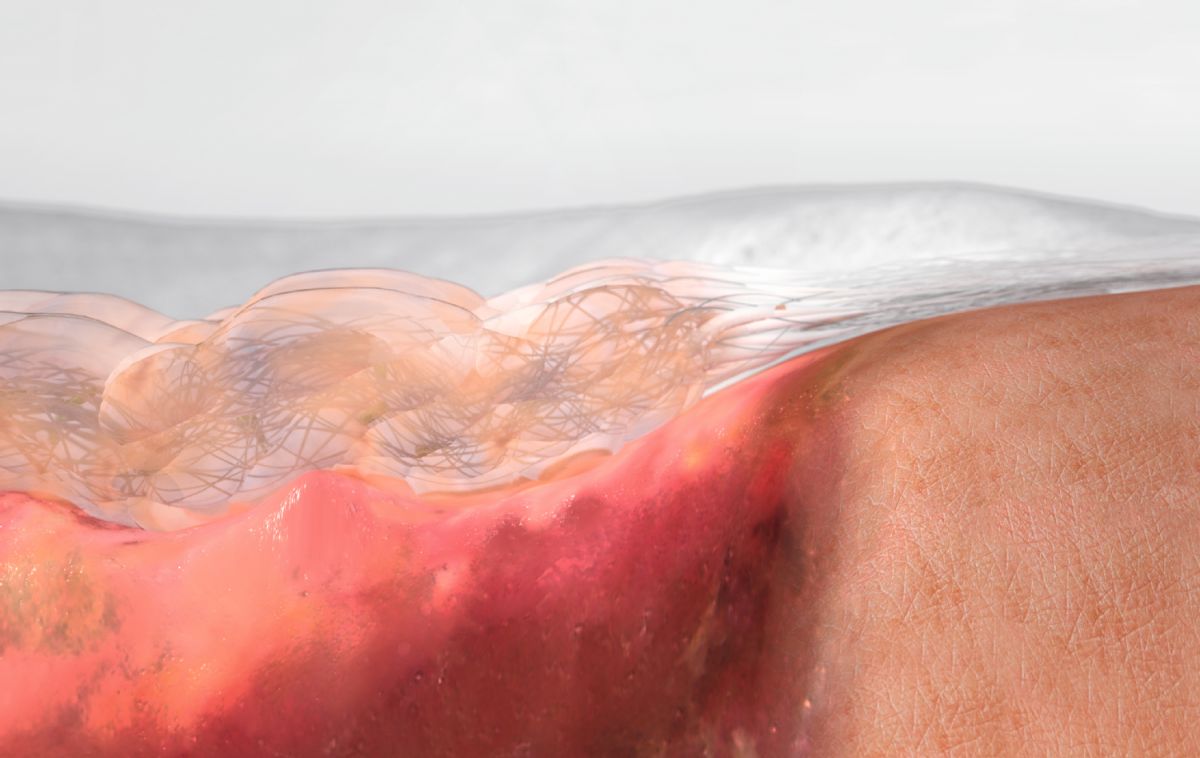
Suprasorb Liquacel reduces the risk of maceration by vertically absorbing and transferring exudate to the secondary dressing. Suprasorb Liquacel gels on contact with wound exudate, trapping exudate, debris and bacteria.
As Suprasorb Liquacel is easy to cut when dry, it is easy to apply/pack. It is equally simple to remove. When moist, Suprasorb Liquacel can be readily removed in one piece. This minimises the risk of dressing residue being left in the wound.
* The properties mentioned above refer to the current in vitro data.
Product composition
cellulose fibres, cellulose ethyl sulfonate fibreVersions
Dressing
Rope
Fields of application
For the care of acute and chronic superficial or deep wounds with low to high levels of exudate
Indications:
- lower leg ulcers
- pressure ulcers
- diabetic ulcers
- 2nd degree burns
- lacerations, cuts and abrasions
- traumatic wounds and wounds with a tendency to bleed, e.g. after surgical or mechanical debridement
- skin graft donor sites
Wound healing stages:
- Exudation phase
- Granulation phase
Properties
- helps to protect the wound edge and the skin surrounding the wound
- effective exudate management promotes the healing process
- simple to use when moist as can be removed in one piece
- vertical absorption and transfer of exudate to the secondary dressing
To note
- When dry, Suprasorb Liquacel* can easily be cut to the size of the wound. The dressing should overlap the wound edges by approx. 1 cm.
- Loosely pack deep wounds to 85% with rope and leave approx. 2.5 cm extending past the wound edges.
Dressing change interval:
- To be determined by the attending physician based on the wound condition, level of exudate, and secondary dressing used, but no longer than 7 days.
*) The properties mentioned above refer to the current in vitro data.